Timing matters: How optimal timing of therapies in COVID-19 patients can mean life or death
Article by Alexis Phillips & David Fajgenbaum, MD, MBA, MSc
Infographics by Ania Korsunska
The COVID-19 pandemic is a global threat that has claimed over 2.16 million lives as of the end of January of 2021 (1). In the majority of individuals, SARS-CoV-2 infection causes mild to moderate flu-like respiratory symptoms and patients recover well. However, some patients experience more severe symptoms, including progression to a severe hyperinflammatory phase (a “cytokine storm”) leading to acute respiratory distress syndrome (ARDS) and progressive multiorgan failure.
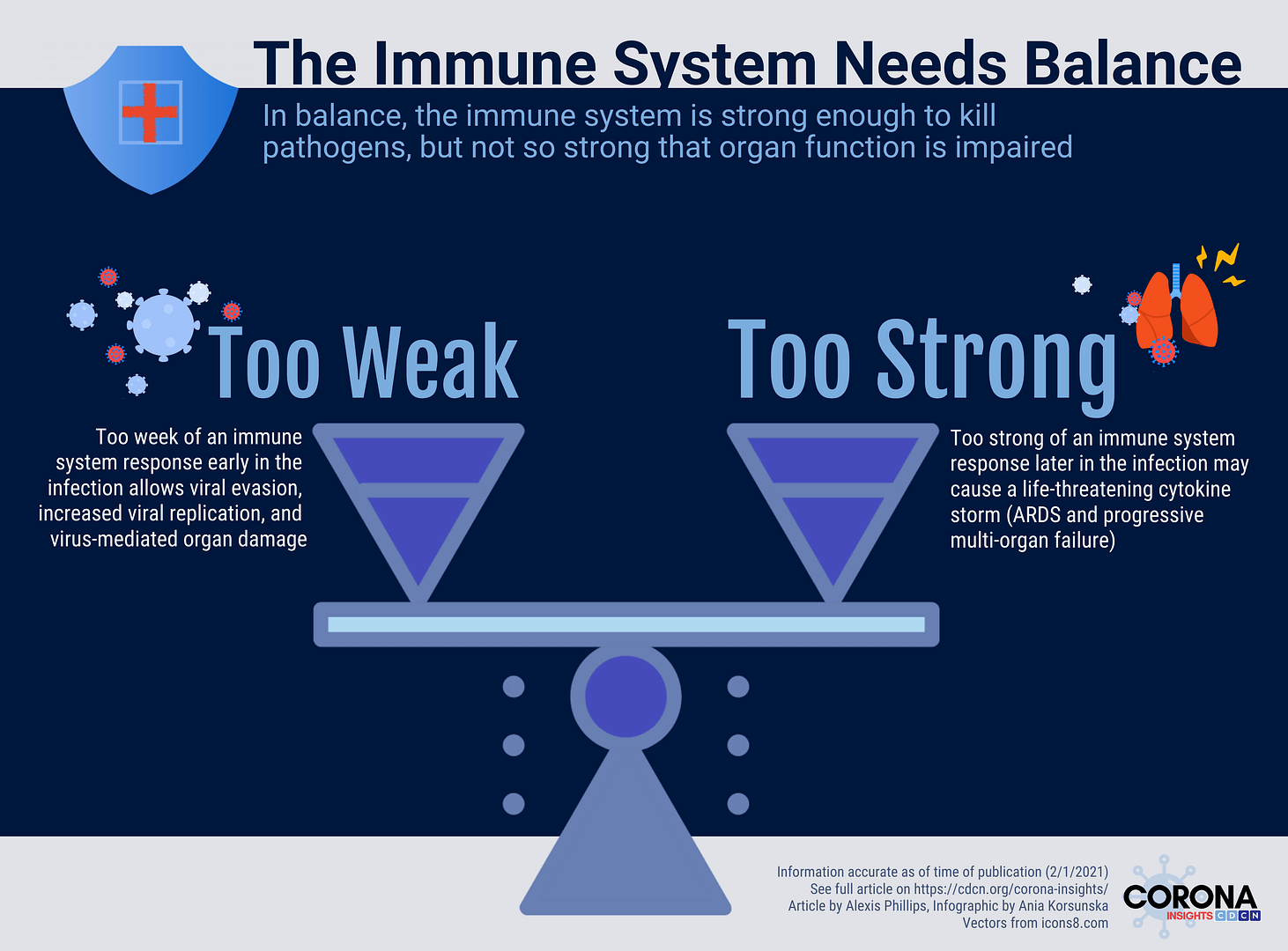
In all viral infections, there is a conflict between the virus and the patient’s antiviral immune response. Too weak of an immune response and the virus evades the immune system, causing damage throughout the body. Too strong of an immune response and the immune system causes damage to healthy organs throughout the body. While many questions remain, currently available research suggests that COVID-19 is capable of causing both (1) too weak of an immune response early in the disease course that allows viral evasion and (2) too strong of an immune response later in the infection that may cause a life-threatening cytokine storm (2).
The CORONA (COvid Registry of Off-label and New Agents) registry has allowed us to identify global trends in the use of repurposed COVID-19 therapies that correspond to the two phases of the disease. We noticed, for example, high use of interferon, an immunostimulant, outside of the United States, as well as high use of dexamethasone, an immunosuppressant, in spite of guidelines advising against it early in the pandemic. These observations led us to hypothesize early on that different types of drugs can be selectively administered to COVID-19 patients to improve antiviral immunity or prevent cytokine storms, depending on the stage or manifestation of disease. Here, we provide a summary of studies that suggest the importance of appropriate timing of antiviral and immunomodulatory therapy in patients with COVID-19.
Early reinforcement of the antiviral response
The early response to infection with SARS-CoV-2 involves activation of many types of immune cells that act in concert to suppress viral replication and promote viral clearance. These cells produce proinflammatory cytokines, including interleukin (IL)-1 Beta, TNF-alpha, IL-6, and type-I interferon (IFN). A robust early response is critical to preventing severe disease later on. Studies indicate that a high viral load caused by an inability to suppress the virus is associated with a higher risk of severe COVID-19 and COVID-19 related cytokine storms (3). This has led researchers to hypothesize that antiviral and immunostimulatory drugs (such as interferon) may effectively treat COVID-19 and prevent cytokine storms when administered early in the infection.
Type-I IFNs are key mediators of the short-term (innate) and long-term (adaptive) antiviral response that may have important treatment implications for patients with COVID-19. Several studies show that weak IFN signaling early in the disease is a feature of many of the most severe cases of COVID-19 (4, 5, 6). An impaired IFN response can be caused by neutralizing antibodies against type-I IFN, found in approximately 10% of patients with severe COVID-19 in one study (7). In another study of 659 severely ill COVID-19 patients, researchers uncovered loss of function genetic mutations causing weakened type-I IFN-dependent immunity in 3.5% of patients (8). These data suggest that IFN is critical to appropriate control of COVID-19 and that some patients may benefit from supplementation with exogenous IFN early in the infection.
Studies on the use of IFN in hospitalized patients with COVID-19 have shown mixed results. In a double-blind randomized controlled trial of 101 hospitalized COVID-19 patients not on mechanical ventilation, treatment with an inhaled formulation of interferon-beta-1a for 14 days resulted in a 79% reduction in the odds of developing severe disease or dying (P = 0.046) and more than three-fold greater odds of clinical improvement 28 days after randomization (9). Patients were also more likely to recover ambulatory ability and had greater reductions in shortness of breath. In the SOLIDARITY randomized controlled trial, hospitalized patients with COVID-19 who were given subcutaneous or intravenous IFN showed a trend toward increased mortality. This trend was even more pronounced among patients receiving invasive respiratory support on randomization (RR 1.40 [99% CI 0.82-2.40]) relative to patients who were not on ventilators (RR 1.11 [99% CI 0.84 – 1.45]) (10). While the mode of drug administration (inhaled vs. subcutaneous/ intravenous) may have influenced the different outcomes of these studies, part of the response may also be accounted for by the inclusion of patients with severe or critical disease in the SOLIDARITY trial. Further research into the administration of IFN to non-hospitalized patients with COVID-19 is warranted.
Late inhibition of cytokine storms
Some COVID-19 patients experience a deadly cytokine storm marked by rapid clinical deterioration around 7-10 days after the onset of symptoms (11). Researchers have hypothesized that the use of immunosuppressive and anti-inflammatory medications, such as corticosteroids, Janus kinase-signal transducer and activator of transcription (JAK-STAT) inhibitors, and intravenous immunoglobulin may be useful in treating patients with COVID-19 cytokine storms.
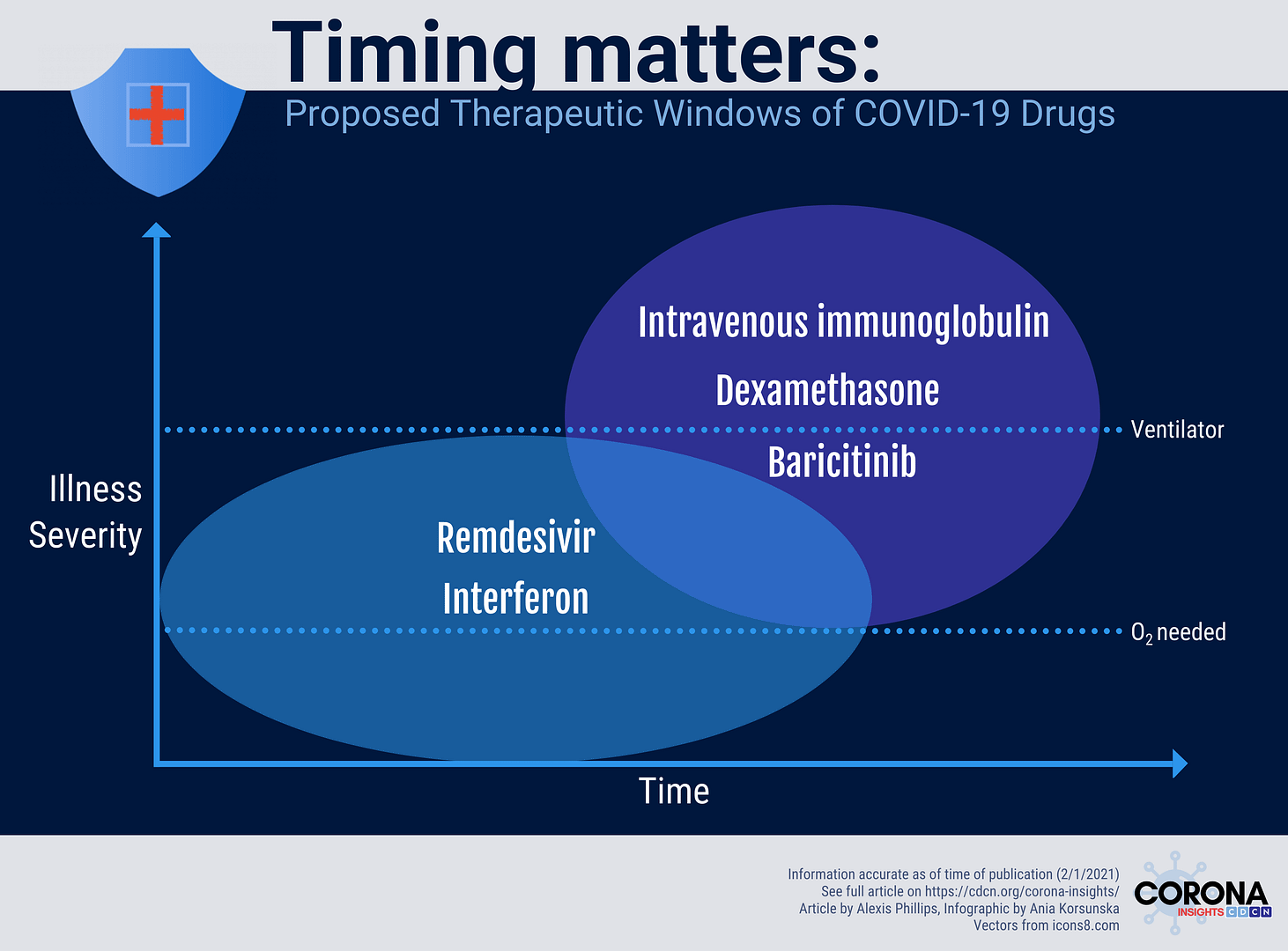
While the use of corticosteroids in COVID-19 patients was initially debated, currently available data provide strong support for its use in patients with inflammation-mediated organ dysfunction. Initial treatment guidelines did not recommend corticosteroids, like dexamethasone, due to early results showing that corticosteroids can contribute to prolonged viral shedding and secondary bacterial infections without survival benefit in the SARS outbreak of 2003 (12). These guidelines were later reversed after publication of the RECOVERY trial (13). This multicenter, controlled, open-label trial found dexamethasone to be associated with lower 28-day mortality in patients on ventilators or receiving oxygen, but not among those who were not receiving respiratory support. The results of this study suggest that dexamethasone may contribute to a reduction in inflammation-mediated lung injury among patients receiving respiratory support.
Baricitinib is a small molecule JAK inhibitor that is currently under investigation for treatment of severe COVID-19. The JAK-STAT pathway may contribute to the inflammatory phenotype of severe COVID-19 by upregulating the activity of inflammatory cells and cytokines. A double-blind randomized controlled trial of 1,033 hospitalized adults with COVID-19 (Adaptive COVID-19 Treatment Trial (ACTT-2)) evaluated the safety and effectiveness of baricitinib plus remdesivir for reducing time to recovery (14). Preliminary results of the double-blind randomized controlled trial revealed that the combination of baricitinib and remdesivir resulted in a statistically significant decrease in the time to recovery relative to the remdesivir and placebo group. The results of this study were responsible in part for inspiring the emergency use authorization of baricitinib by the FDA (15).
Intravenous immunoglobulin (IVIg) is another therapy that can be used to downregulate the immune system during the hyperinflammatory phase of COVID-19 (16). In a double-blind randomized controlled trial published in October of 2020, researchers studied the effects of IVIg on clinical outcomes in 30 out of 59 patients with severe COVID-19 that was refractory to initial therapy (17). The researchers found that the in-hospital mortality rate was significantly lower in the IVIg group compared to the control group (P = 0.025). This effect remained significant even after adjusting for several other variables that may have had an effect on mortality (P = 0.042). This study provides support for a larger trial to study the use of IVIg to reduce inflammation-mediated organ damage and mortality in patients with severe COVID-19.
The importance of trials that investigate the therapeutic window
Emerging data on COVID-19 highlight the importance of administering the right medications, to the right patients, at the right time. There remains an urgent need for further exploration of the natural history of COVID-19, effective treatments that can be used, and how to optimize timing of treatments to increase likelihood of success.
Data accurate as of date of publication (2/1/2021). Featured image Illustration by Julia from Icons8. Other icons in infographics also from icons8.com.
Acknowledgments: Special thanks to Johnson Khor
Disclaimer:
CORONA Insights and the CORONA Treatment Registry are for informational and exploratory purposes only and are not intended to be used as medical advice. This site is intended to facilitate the exploration of therapies mentioned in COVID-19 medical literature. Users assume full responsibility for use of the information on this site and understand and agree that the CDCN and its third party content providers are not responsible or liable for any claim, loss, or damage (including personal injury or wrongful death) resulting from its use. Reliance on any information provided by the CDCN or third party content providers is solely at your own risk. Our extractors do not assess the scientific merits of these results and conclusions. We disclaim any warranty concerning the accuracy, timelessness, and completeness of information on this site. This site also contains links to external sites. The CDCN is not responsible for the content and does not make any representations regarding their content, timeliness, or accuracy.