Drug repurposing for Castleman Disease
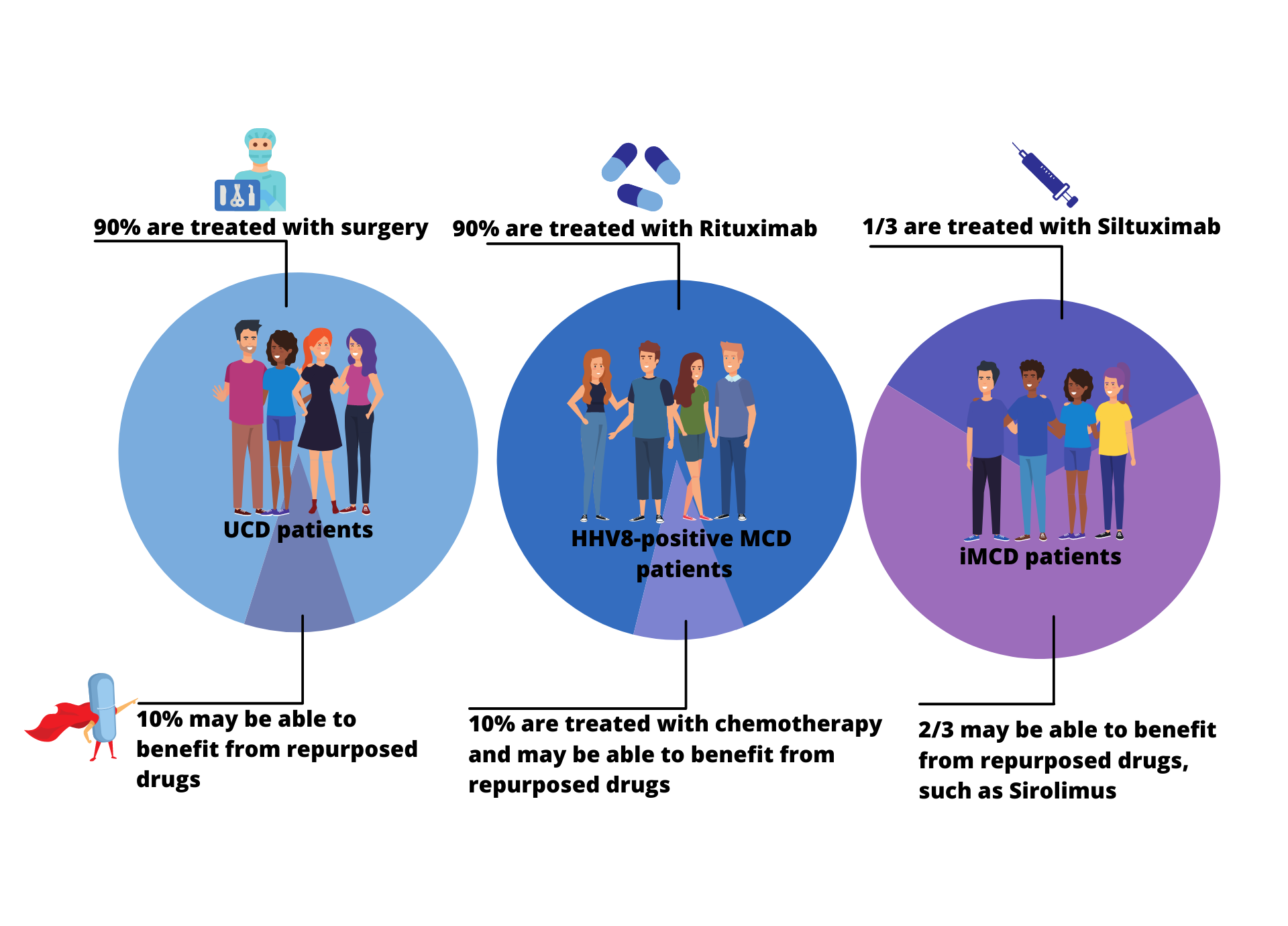
Though only one treatment is FDA approved for one subtype of Castleman disease, several treatment approaches are well established for the various subtypes of Castleman disease. Approximately 90% of UCD patients are successfully treated with surgical excision and approximately 90% of HHV8-positive MCD are successfully treated with rituximab/Rituxan, which is not approved for HHV8-positive MCD but is a great example of effective drug repurposing. About one-third to one-half of iMCD patients are successfully treated with siltuximab/Sylvant, which is FDA-approved for iMCD. For the remaining UCD, HHV8-positive MCD, and iMCD patients that do not benefit from these therapies, several drugs have been identified as potential repurposed treatments and extensive research is underway to uncover more.
The CDCN’s drug repurposing approach has led to the successful identification of drug targets and drugs that can help CD patients. The CDCN’s co-founder and executive director, Dr. David Fajgenbaum, also a patient with iMCD, began testing one of these drugs on himself in 2014 and is currently in his longest remission ever (read more about his “Chasing My Cure” story here). See below for details about the CDCN’s successful drug repurposing efforts.
