The CDCN created an innovative roadmap to accelerate CD research and treatment discovery by leveraging and integrating the entire community of stakeholders – patients, loved ones, physicians, and researchers.
Building a Community
Together, the community identifies and prioritizes high-impact research questions. It then identifies and recruits the most qualified researchers to conduct each study. In parallel, patients are empowered to fight back by fundraising and providing their biospecimens and clinical data for analysis in these studies. This approach democratizes research to identify the most clinically relevant and pressing questions; any idea can be translated into a study rather than limiting ideas to those conceived of by researchers in grant applications. This approach can be broken into eight critical steps and serves as a model for other rare diseases.
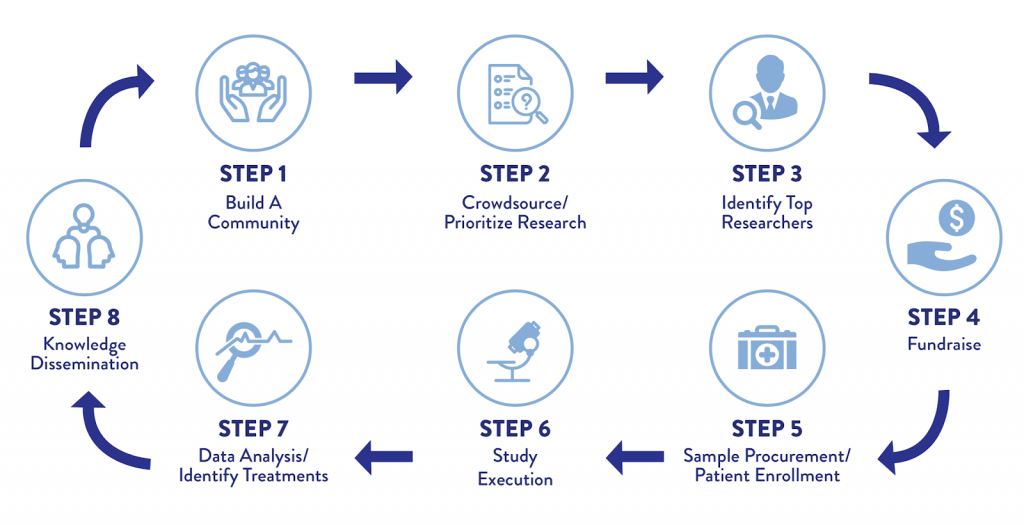
Crowdsourcing and Prioritizing Research Studies
Physicians, researchers, patients, and loved ones each contribute key perspectives and skills to the table. By working together, the group poses unique questions that set the research strategy of the organization. Integrating the input and ideas of each of these groups allows collaboration to prioritize the most clinically meaningful questions and research approaches to answering them.
Identifying Top Researchers
The CDCN recruits and engages leading researchers to study the identified research priorities and aligns researchers’ skill sets with specific studies.
Fundraising
Our very low operating costs allow us to maximize the impact of every dollar raised because we are largely sustained by a volunteer workforce. Over 90% of every donation goes directly to research – where it makes the most meaningful impact to CD patients.
Procuring Samples and Enrolling Patients
Identification of the proper study subjects is essential to the progress of a clinical trial or laboratory study. Each study on the International Research Agenda has a detailed strategy to obtain samples (e.g., blood, saliva, lymph node tissue, etc.). To support wider and future research efforts, the CDCN recently established a biobank for the collection and sharing of biospecimens with the entire research community.
The ACCELERATE Natural History Registry, allows patients anywhere in the world to consent online for medical record acquisition and extraction. Together, its biobank and ACCELERATE allow the CDCN to collect and correlate key clinical information with biospecimens. The CDCN also procures biospecimens through collaborations with physicians, researchers, and corporate partners.
Executing Studies & Identifying Treatments
Once all pieces are brought together, the study is ready to begin. However, since many of the research projects that the CDCN leads have multiple collaborators, our team continues to stay involved throughout the execution phase. By fostering connections between the multiple collaborators, the CDCN promotes cooperation that improves outcomes and increases the speed of project completion. We place special emphasis on identifying new drug targets for treating CD. One strategy is aimed at identifying FDA-approved drugs as candidates for off-label use in CD. These treatments are captured in the ACCELERATE Natural History Registry to gain insights into how effective they are in a real-world setting. When promising drugs are identified, clinical trials are planned to rigorously assess efficacy.
Disseminating Knowledge
The CDCN promptly publishes results of research studies in order for the broader CD community to benefit from findings. A well informed community is better able to identify and prioritize the next round of high-impact research.
And the cycle continues: more individuals join, new research ideas are shared (inspired by the findings), and greater progress towards the CDCN’s mission is accomplished.
Learn more about the CDCN’s Collaborative Network Approach, published in the Portland Press.
Click to donate to high-impact CD research or get involved.
