Drug Repurposing
Drug repurposing involves identifying FDA-approved drugs that may be effective for diseases they’re not currently approved for. We believe that this is a much more efficient process than new drug research and development for rare diseases like Castleman disease. The CDCN is committed to discovering new uses for existing drugs to save patients’ lives and helping support other patient organizations in their drug repurposing efforts.
Fast facts about rare disease treatment:
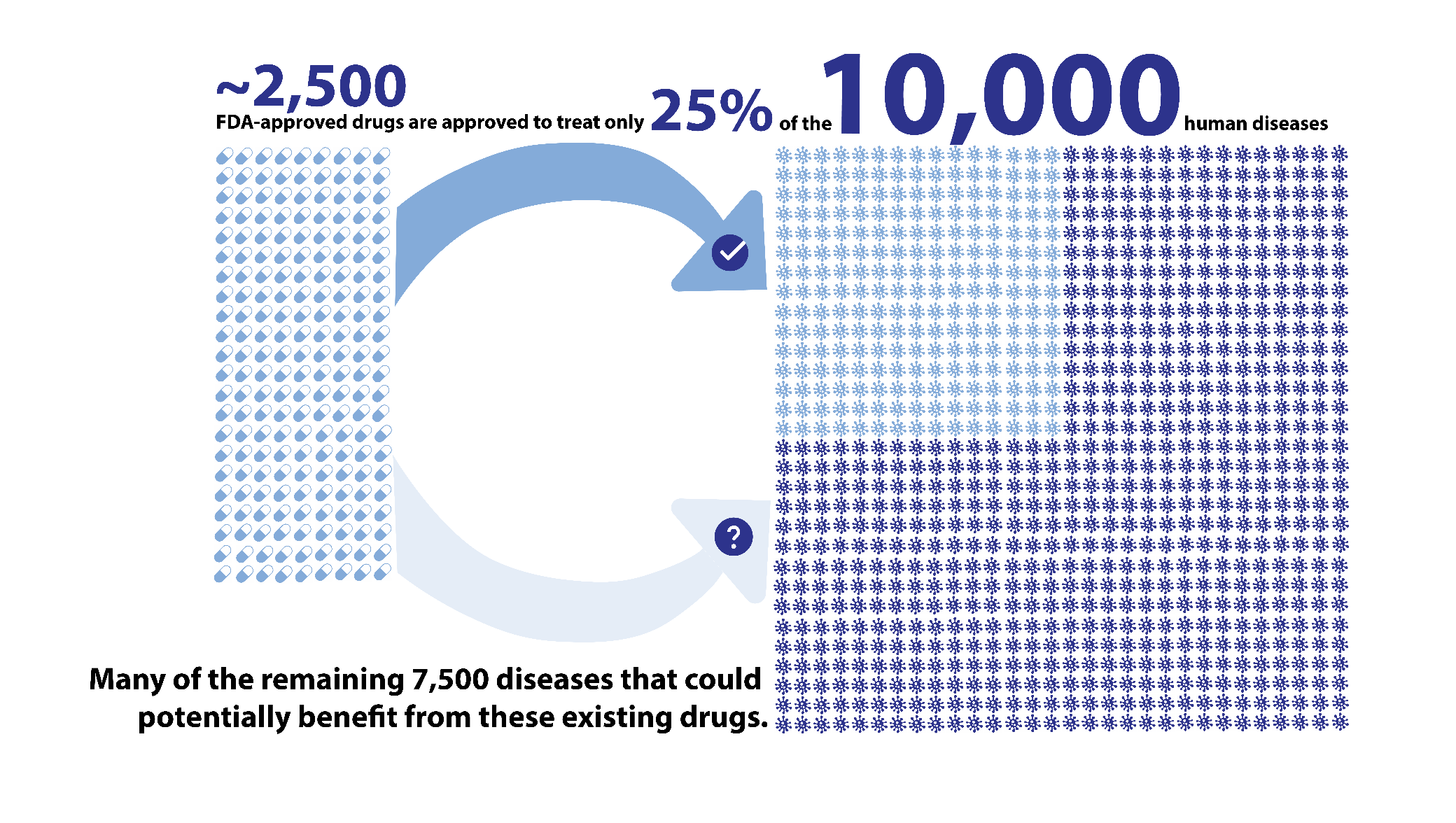
- Currently, there are ~2,500 FDA approved drugs that treat 25% of the ~10,000 human diseases
- No treatments exist for over 7,000 diseases, many of which are rare and deadly
- Developing a new drug can cost $2-3 billion and can take 13-15 years before becoming approved for widespread use
- New drug development is also very risky. The drug may turn out to be unsafe or ineffective at hitting its target. Repurposed drugs have a known safety profile and established effect.
- Many rare diseases are heterogeneous and/or idiopathic.
- By definition, rare diseases do not affect a large patient population and often do not present enough of a commercial incentive for pharmaceutical companies to develop new treatments.
 Given that centuries will be needed to develop a new drug for all rare diseases, drug repurposing provides a potential alternative solution. The basic idea of drug repurposing is that many diseases share similar defects and thus may benefit from the same treatments. Once a treatment has been FDA-approved and shown to be safe and effective for at least one disease, it is more likely to be safe and potentially effective in a new disease area than new, untested compounds. Research is required to identify and match potential treatments to specific conditions. This approach is often much faster and cheaper than new drug development.
Given that centuries will be needed to develop a new drug for all rare diseases, drug repurposing provides a potential alternative solution. The basic idea of drug repurposing is that many diseases share similar defects and thus may benefit from the same treatments. Once a treatment has been FDA-approved and shown to be safe and effective for at least one disease, it is more likely to be safe and potentially effective in a new disease area than new, untested compounds. Research is required to identify and match potential treatments to specific conditions. This approach is often much faster and cheaper than new drug development.
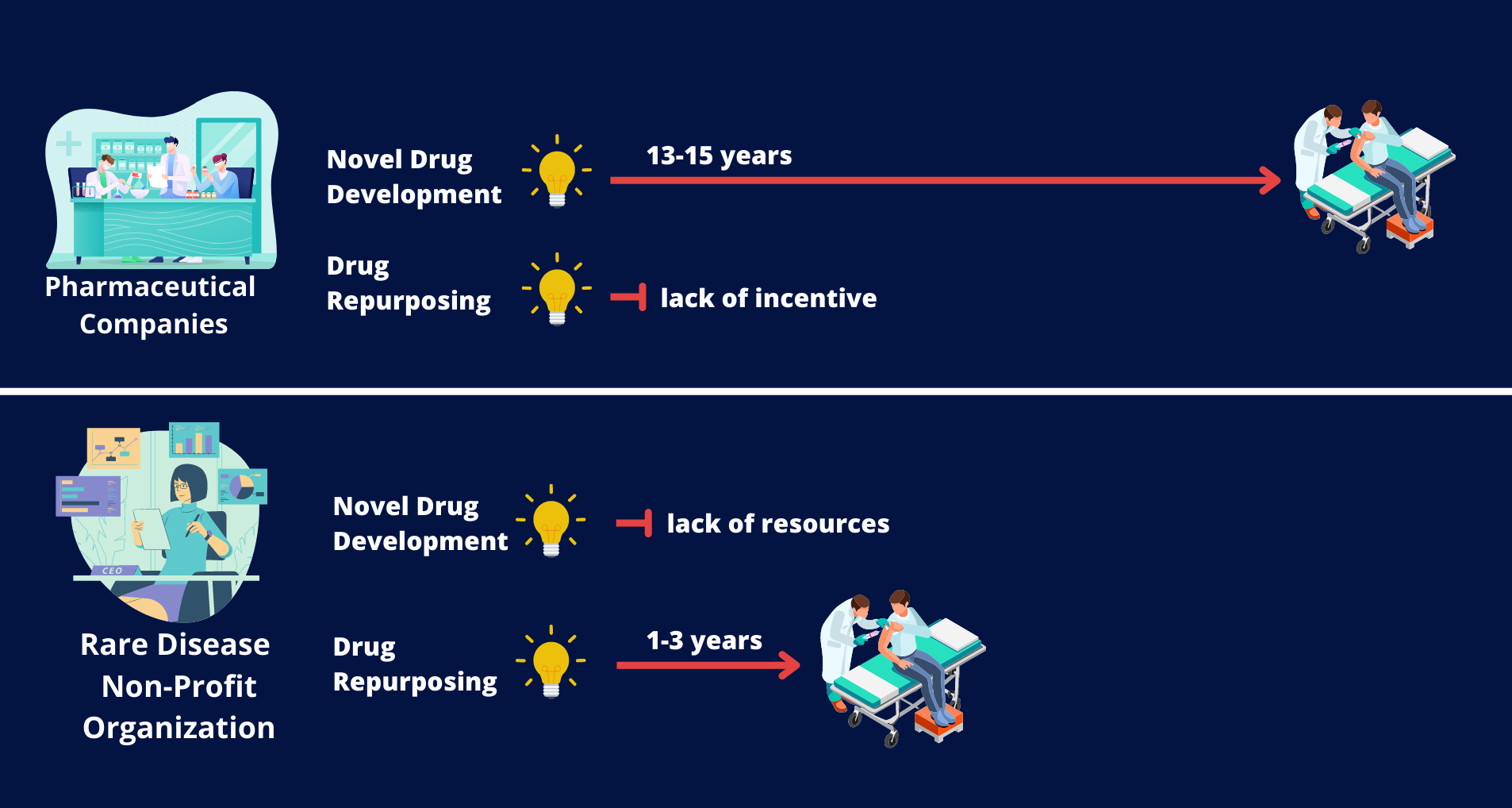
Thus, redirecting more funding and research towards maximizing the uses of existing treatments, rather than focusing solely on the expensive, time-consuming and risky development of novel drugs, is potentially one of the fastest and most effective ways to get treatments to patients with rare diseases.
