Promising treatments uncovered in CORONA (the COvid Registry of Off-label and New Agents)
By Alexis Phillips
We are excited to share key highlights from the ongoing CORONA (COvid Registry of Off-label and New Agents) project, our contribution to the global fight against COVID-19. CORONA is a publicly available central repository of treatments administered to COVID-19 patients dating back to the start of the global pandemic. The registry allows clinicians, researchers, and other interested individuals to uncover trends in the use and reported the effectiveness of therapies. Most importantly, it’s being used to facilitate the identification of promising therapies that should be further investigated in clinical trials.
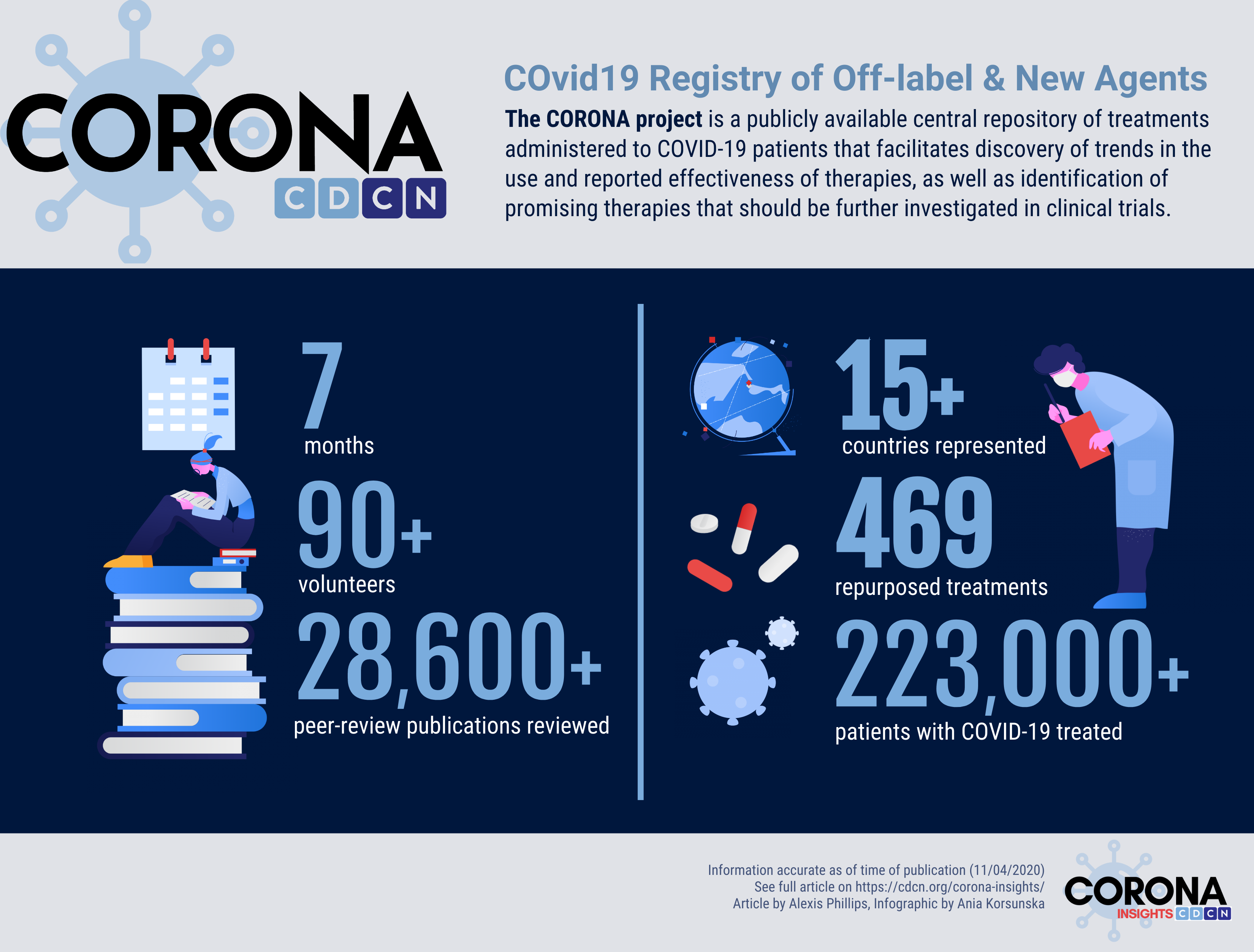
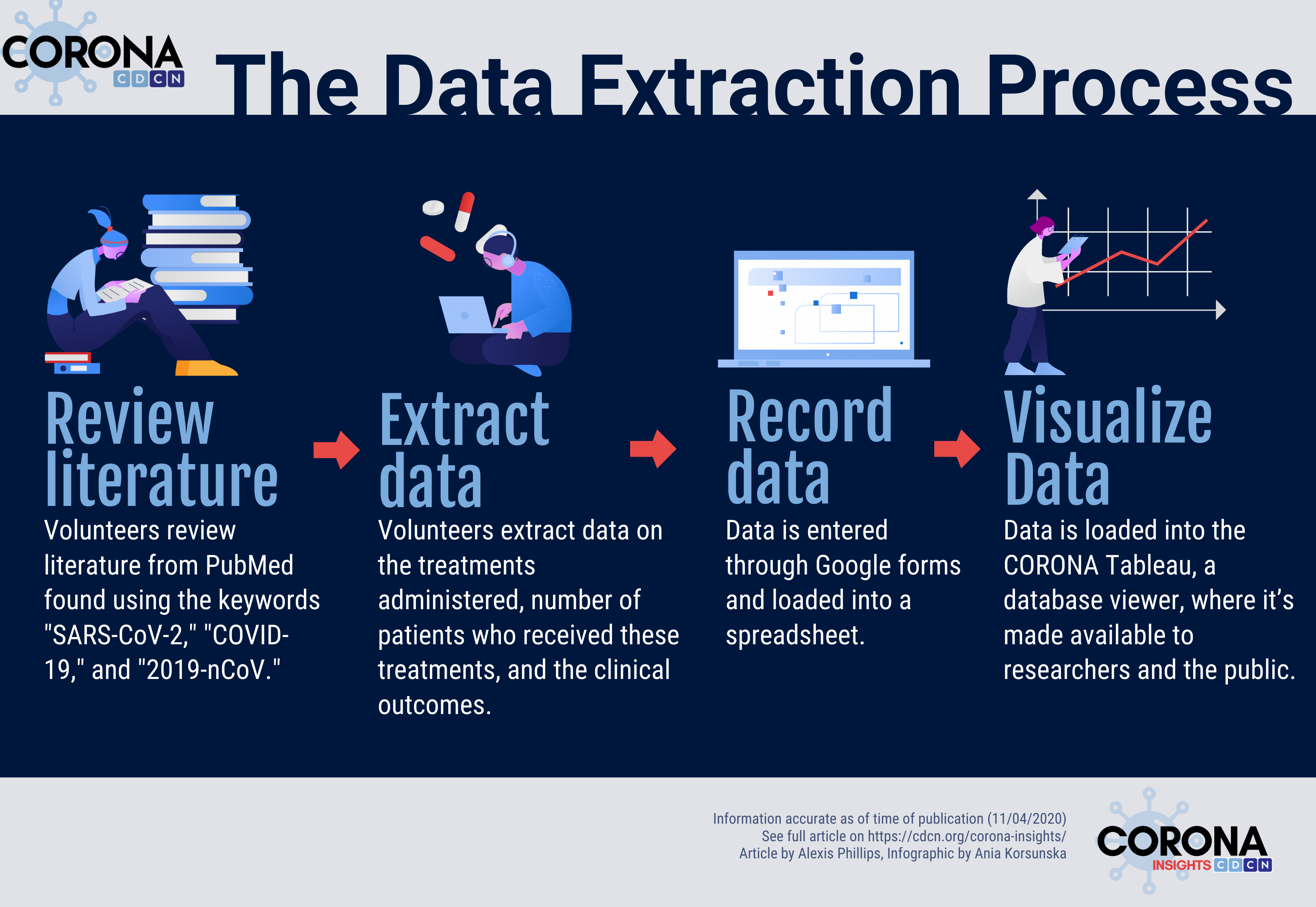
To build CORONA, our diverse team of over 90 volunteers has worked diligently over the past seven months to review more than 28,600 papers published on COVID-19 and extract information on over 469 treatments and treatment categories given to more than 223,000 COVID-19 patients. For each paper that describes treatments administered to COVID-19 patients, our volunteers extract the treatment(s) administered, the number of patients who were treated with each drug, and the reported clinical outcomes of the patients who were treated. We’ve also partnered with the FDA to ensure that our clinical outcomes are also reported in their CURE ID app (1).
Moving forward, we plan to share periodic posts about the key insights and implications of this important work. Today, we wanted to highlight some of the most promising COVID-19 treatments.
9 Therapies that Stand Out and Show High Potential:
We have analyzed the database to identify a list of drugs that stand out as showing high potential to be effective in treating COVID-19 patients based on the frequency of administration and the proportion of patients who were reported to improve. When available, we gave additional weight to drugs that show promise in randomized controlled trials.
To provide context, the course of SARS-CoV-2 infection is mild in most individuals and starts with viral entry and replication in the cells of the respiratory tract. In some, the illness progresses to include pneumonia, and in a smaller number of patients, COVID-19 can cause a life-threatening cytokine storm. A cytokine storm is an excessive immune response that can lead to multiorgan failure.
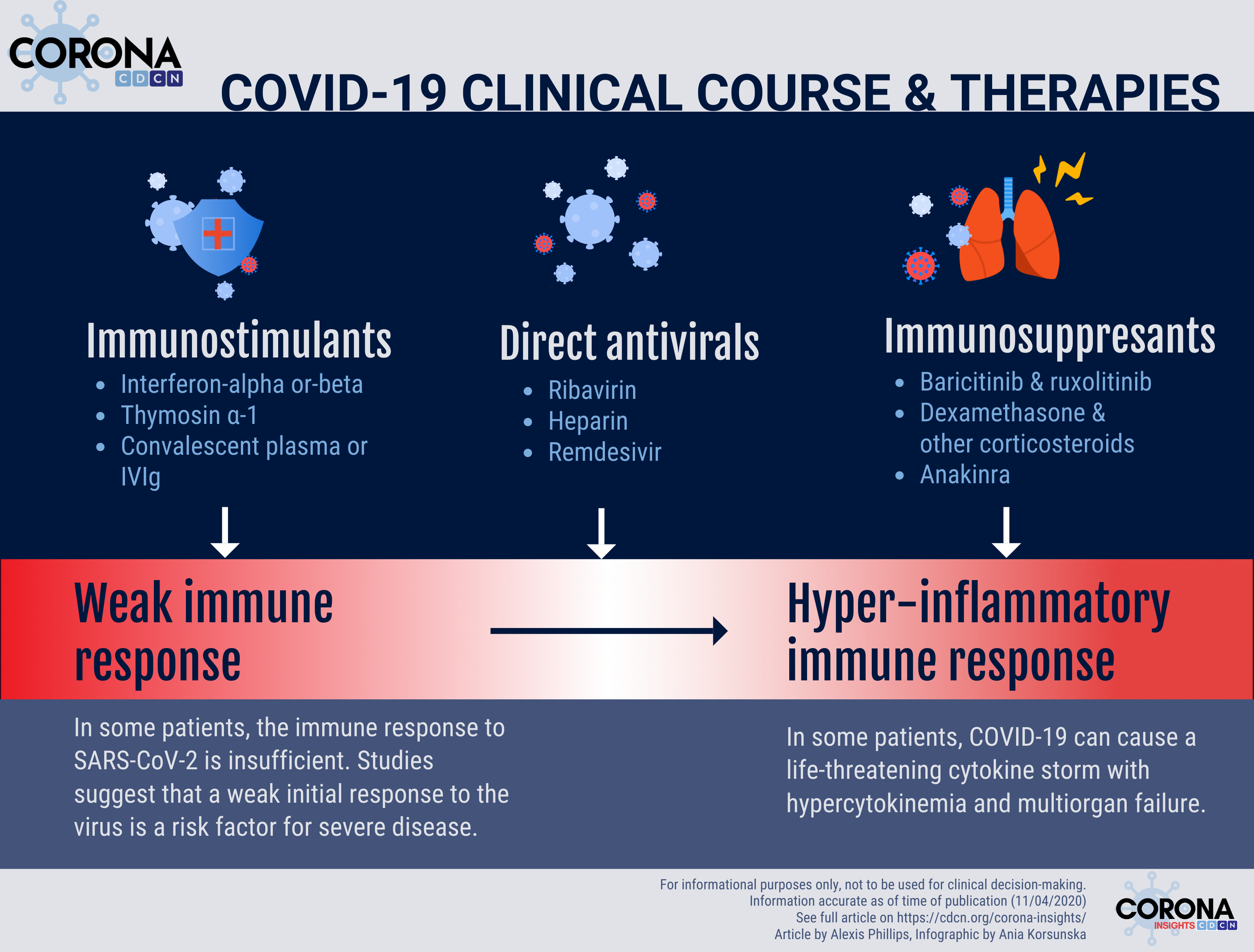
COVID-19 treatments can generally be broken up into drugs that directly target the virus, drugs that boost the immune system to better fight the virus, and drugs that suppress the immune system so it doesn’t spiral out of control (cytokine storm). The following list is ordered by the presumed treatment category, not rank order:
Drugs that directly target viral replication and transmission throughout the body:
- Ribavirin – Ribavirin has several mechanisms that interfere with the replication of DNA and RNA viruses (2). In vitro studies have demonstrated antiviral activity against SARS-CoV-2; however, high doses of ribavirin are required for the drug to be effective, and high doses carry a significant risk of toxicity. To balance clinical efficacy and toxicity risk, available data suggest that ribavirin may be best administered in low doses and in combination with other therapies (3).
- Heparin – As an antithrombotic agent, heparin prevents blood clots that can be associated with hyperinflammatory states like COVID-19 cytokine storms. Heparin is also known to have anti-inflammatory effects both in the vasculature and the airway by modulating the activity of pro-inflammatory proteins (IL-8, MBP, PGF4, CD11b/CD18) (4). Based on much more limited experimental data, heparin may have direct anti-SARS-CoV-2 activity by binding to viral spike protein and preventing viral entry into host cells.
- Remdesivir – Remdesivir is an antiviral medication that interferes with viral RNA replication. While in vitro and animal studies consistently demonstrate anti-SARS-CoV-2 activity and clinical benefit, randomized controlled trials of remdesivir in hospitalized COVID-19 patients have shown mixed results. In the ACTT-1 trial, remdesivir was associated with a significantly shorter time to recovery in hospitalized patients with COVID-19 (5). However, in the more recently published Solidarity trial that separately evaluated four antiviral drugs (remdesivir, hydroxychloroquine, lopinavir/ritonavir, and interferon-β1a), remdesivir was found to have no significant effect on mortality, initiation of ventilation, and duration of hospital stay (6). It is important to consider that the effectiveness of direct-acting antivirals typically decreases with delays in treatment initiation. Both the ACTT-1 and Solidarity trials test the effect of remdesivir in patients who were hospitalized and therefore may have been outside of the optimal therapeutic window. In October 2020, remdesivir became the first FDA-approved treatment for COVID-19 (7).
Drugs that boost too weak of an immune response to viral infection:
- Interferon-alpha or interferon-beta – Interferon (IFN)-alpha and interferon-beta are endogenous cytokines that the immune system uses to initiate an effective antiviral response. Data suggest that some patients with COVID-19 may go on to have a cytokine storm because they did not produce enough interferon early in the disease course to control the infection (8). Researchers have therefore inferred that some COVID-19 patients may benefit from therapy with exogenous IFN early in the infection. An open-label, randomized phase II trial has found a reduction in duration of viral shedding in COVID-19 patients who received interferon-beta within 7 days of symptom onset (9).
- Thymosin-ɑ1 – Thymosin-ɑ1 is an endogenous immune regulator that enhances cell-mediated immunity and is also believed to help induce antibody production. These immunostimulatory actions may be helpful for neutralizing the virus (10).
- Convalescent plasma or IVIg – Convalescent plasma from individuals who have recovered from SARS-CoV-2 infection may contain antibodies against SARS-CoV-2 that can help other patients who are recently infected to recover faster. While convalescent plasma has not been evaluated in randomized controlled trials, available retrospective data suggest that convalescent plasma may be associated with a reduction in mortality in critically ill patients, increased clearance of SARS-CoV-2 RNA, and improvement in clinical symptoms (11).
Drugs that inhibit an excessive immune response to SARS-CoV-2 (COVID-19 “cytokine storm”):
- Baricitinib or ruxolitinib – Baricitinib and ruxolitinib are both JAK1/2 inhibitors that appear to be promising for patients with severe COVID-19 cytokine storms (12). Many of the cytokines responsible for cytokine storms (including IL-6 and interferon) signal through the JAK1/2 signaling pathway and can be suppressed with these drugs in patients with hyperinflammatory conditions similar to COVID-19. A recent randomized controlled trial found that the administration of baricitinib plus remdesivir was associated with a reduction in recovery time compared to remdesivir alone among hospitalized COVID-19 patients (13).
- Dexamethasone and other corticosteroids – Corticosteroids may help to prevent inflammation-mediated lung injury in patients with COVID-19 by suppressing inflammation when given late in the disease course. However, they may be ineffective or harmful if administered early, when an inflammatory response is needed to fight infection. The RECOVERY trial was a randomized controlled trial of hospitalized COVID-19 patients that found dexamethasone to be associated with lower 28-day mortality in patients on ventilators or receiving oxygen, but not among those who were not receiving respiratory support (14).
- Anakinra – Anakinra is an interleukin-1 (IL-1) inhibitor. IL-1 is a key cytokine in the inflammatory immune response that may be increased in COVID-19 cytokine storms. In particular, elevated IL-1β is a key driver of acute respiratory distress syndrome (ARDS), a life-threatening lung injury seen in severe cases of COVID-19 (15). Several studies are underway of anakinra and other drugs that inhibit IL-1.
We have shared this list of promising agents with collaborators and will continue to advocate for further large, well-powered randomized controlled trials of these agents and others. There are a few important considerations when interpreting the data. First, many of the studies in CORONA are observational studies or case reports that may be subject to publication bias and cannot be used to establish causal relationships between treatments and outcomes. The studies that compare outcomes between different experimental groups are also designed differently and include patients from different backgrounds, clinical environments, and countries, with different degrees of clinical severity. Therefore, results cannot be easily compared between treatments or trials. It is also important to note that CORONA reviewers make a subjective assessment when establishing the CURE ID response. In spite of these caveats, CORONA reveals interesting trends that can be used to inspire further studies.
Reviewing the published literature on COVID-19 has been a massive undertaking made possible by the contributions of many dedicated volunteers. If you would like to join our team, please reach out to Johnson Khor: johnson.khor@pennmedicine.upenn.edu.
Data accurate as of date of publication (11/4/2020). Featured image Illustration by Julia from Icons8. Other icons in infographics also from icons8.com.
Acknowledgments: Special thanks to Dr. David Fajgenbaum, Ania Korsunska, Mileva Repasky, Lauren Marek, Meggie Goodridge, & Johnson Khor.
Disclaimer:
CORONA Insights and the CORONA Treatment Registry are for informational and exploratory purposes only and are not intended to be used as medical advice. This site is intended to facilitate the exploration of therapies mentioned in COVID-19 medical literature. Users assume full responsibility for use of the information on this site and understand and agree that the CDCN and its third party content providers are not responsible or liable for any claim, loss, or damage (including personal injury or wrongful death) resulting from its use. Reliance on any information provided by the CDCN or third party content providers is solely at your own risk. Our extractors do not assess the scientific merits of these results and conclusions. We disclaim any warranty concerning the accuracy, timelessness, and completeness of information on this site. This site also contains links to external sites. The CDCN is not responsible for the content and does not make any representations regarding their content, timeliness, or accuracy.