12/16/2019 Another amazing year for the CDCN has passed! Check out some of our top highlights of the year below. All these accomplishments have only been possible due to the support of hundreds of patients, loved ones, physicians, researchers, team members, and community members from all over the world.
1) Partnership with the Chan Zuckerberg Initiative
 The CDCN partnered with the Chan Zuckerberg Initiative in a year-long project to help strengthen and scale the CDCN’s collaborative network approach to advance cures for CDCN and other rare diseases. Our partnership is part of CZI’s Rare as One Project which supports patients to accelerate progress against rare diseases. The CDCN has been helping to test and implement new tech tools that can be utilized by other rare disease communities in hopes of accelerating rare disease research. As part of this project, the CDCN plans to launch private discussion boards for both our patient and loved one community and our physician and researcher community in early 2020!
The CDCN partnered with the Chan Zuckerberg Initiative in a year-long project to help strengthen and scale the CDCN’s collaborative network approach to advance cures for CDCN and other rare diseases. Our partnership is part of CZI’s Rare as One Project which supports patients to accelerate progress against rare diseases. The CDCN has been helping to test and implement new tech tools that can be utilized by other rare disease communities in hopes of accelerating rare disease research. As part of this project, the CDCN plans to launch private discussion boards for both our patient and loved one community and our physician and researcher community in early 2020!
2) Chasing My Cure Book Launch
 CDCN Co-Founder and University of Pennsylvania assistant professor Dr. David Fajgenbaum released his memoir Chasing My Cure in September 2019! Dr. Fajgenbaum hoped that by sharing his story about his battle with Castleman disease (CD) and the lessons he learned along the way, he would help raise awareness and funds for CD and rare disease research. His memoir has been a huge success and has led to features on CNN, Good Morning America, Fox, Newsweek, NYPost, and many more!
CDCN Co-Founder and University of Pennsylvania assistant professor Dr. David Fajgenbaum released his memoir Chasing My Cure in September 2019! Dr. Fajgenbaum hoped that by sharing his story about his battle with Castleman disease (CD) and the lessons he learned along the way, he would help raise awareness and funds for CD and rare disease research. His memoir has been a huge success and has led to features on CNN, Good Morning America, Fox, Newsweek, NYPost, and many more!
The increased visibility of Castleman disease has also resulted in:
- More google searches for Castleman disease in September 2019 than ever before in the history of Google!
- Over 30k unique visitors to CDCN.org in the month after the book launch
- An increase in donations to CDCN for Castleman disease research
- 30+ articles about Dr. Fajgenbaum’s memoir in the USA and hundreds more around the world!
3) Case-series manuscript published in Journal of Clinical Investigation
A new CDCN-supported paper, “PI3K/Akt/mTOR is a Novel Therapeutic Target in IL-6-blockade Refractory iMCD”, was published in the prestigious Journal of Clinical Investigation in August 2019! This ground-breaking study describes using a medication called sirolimus for three patients with idiopathic multicentric Castleman disease (iMCD) who had not improved with the only FDA-approved drug for Castleman disease. This was the first time that sirolimus had ever been tried for Castleman disease, and the paper showed that all three patients benefited from this medication. The research from this paper led to the launch of the sirolimus clinical trial at the University of Pennsylvania and the University of Arkansas for Medical Sciences! The paper has already been cited by articles in top journals and has been viewed over 6,000 times as of December 2019!
4) CDCN.org receives a makeover
We have revamped and launched a new design for CDCN.org! Our team worked with Nucleus Digital to redesign and build our website for the future. We hope that the new website will support and educate our community about Castleman disease and the CDCN and will help facilitate our collaborative research approach to Castleman and rare disease research. We are especially thankful for the support of CDCN Advisory Council member Marc Brownstein who was instrumental in the early stages of our website build. Check out some of our favorite pages below!
5) CDCN and EUSA work together to fight rare diseases
The CDCN has begun a new partnership with the company EUSA Pharma! EUSA Pharma is the maker of the medication siltuximab, the only FDA-approved therapy for idiopathic multicentric Castleman disease. This global biopharmaceutical company has already dedicated significant resources to Castleman disease research and other rare diseases and oncologic research. This year, EUSA Pharma generously sponsored our Patient & Loved One Summit, Quest for a Cure, and our annual working meeting at the American Society of Hematology conference!
6) Quest for a Cure raises $225k+ for CD research
The 5th Annual Quest for a Cure gala was our most successful Quest for a Cure ever! We raised over $230,000 for Castleman disease research—almost twice as much as in 2018! Donations were particularly helped by the support of a generous anonymous donor who matched up to $30,000 of all donations made on the day of the event. We were also pleased to award EUSA Pharma, Quest for a Cure’s headline sponsor, with the 2019 Castleman Warrior Partner of the Year award and the Incorvia family with the 2019 Castleman Warrior Loved Ones of the Year award. Thank you to everyone who helped make our Gala possible and who attended and supported one of our favorite nights of the year!
7) Sirolimus clinical trial has opened
The University of Pennsylvania and the University of Arkansas for Medical Sciences have launched a new clinical trial for patients with idiopathic multicentric Castleman disease (iMCD) that did not benefit from anti-IL-6 treatment (siltuximab or tocilizumab)! This clinical trial evaluates the use of the medication sirolimus for iMCD, and is the first clinical trial for a new drug for Castleman disease in over five years. The trial was made possible based on groundbreaking research supported by the CDCN.
- Learn more about the trial and how to enroll: CDCN.org/trial
- See the clinical trial posting on clinicaltrial.gov
8) ACCELERATE enrolls over 100 patients in 2019!
We have had 108 Castleman disease patients enroll into the ACCELERATE Registry this year! The ACCELERATE registry is a collection of donated de-identified medical records from Castleman disease patients that is used for research to help identify and improve treatment and care for Castleman disease. In the words of David Fajgenbaum, CDCN Co-Founder and Castleman disease patient, “ACCELERATE is our best opportunity to do our parts, as patients, to help ourselves and other patients.”
All Castleman disease patients are encouraged to take just 20-25 minutes to learn more and enroll here.
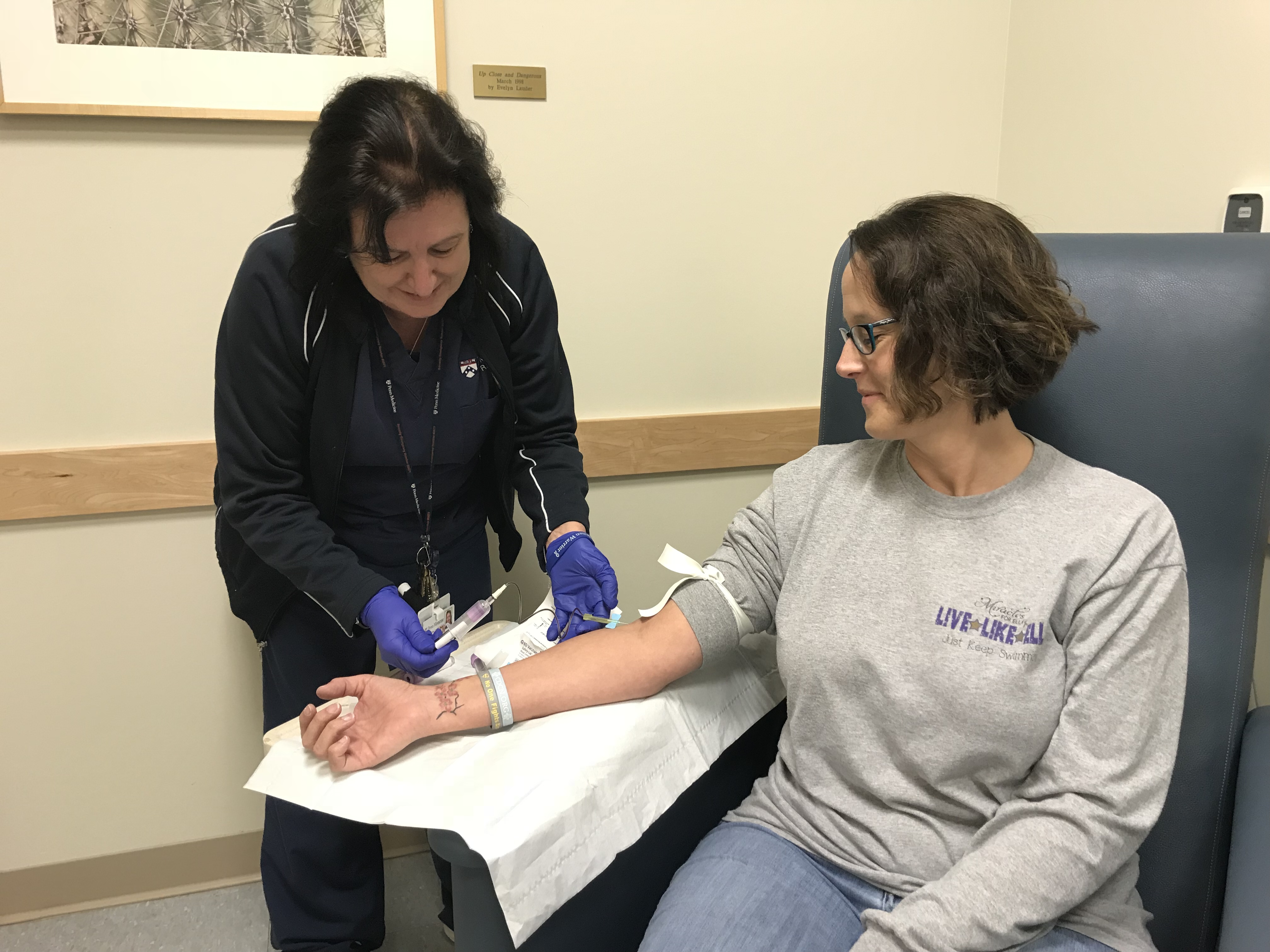 9) Biobank Progress
9) Biobank Progress
This year, 78 samples were donated to the Biobank to be used for high-impact Castleman disease research! We are so grateful to all of the patients, loved ones, and medical team members who helped make these sample donations possible. None of the research progress we have made for Castleman disease would have been possible without these samples.
Blood samples and excess lymph node tissue samples may provide critical information to help better understand and treat this disease. In the words of David Fajgenbaum, CDCN Co-Founder and Castleman disease patient who has donated many samples himself, “The cure is within all of us.”
Please email CastleBank@uphs.upenn.edu or call 267-586-9977 if you’re interested in donating a sample or would like to learn more.
10) CDCN at the American Society of Hematology (ASH) Meeting
Every year, the ASH meeting attracts over 25,000 physicians and scientists across the world. The CDCN held its annual ASH Working Dinner which was attended by 70 leading Castleman disease physicians and researchers from around the world. The dinner served as an opportunity to review new research, ask and answer questions, and discuss ways to improve Castleman disease patient care. Shiela Pierson and Ruth Anne Langan Pai, researchers at the University of Pennsylvania and CDCN volunteers, presented three CDCN-funded research abstracts at the conference this year! Click here to read the abstracts and learn more about our time at ASH!