A working definition of Long COVID is required for optimal clinical trial design
By Hemi Park
Background
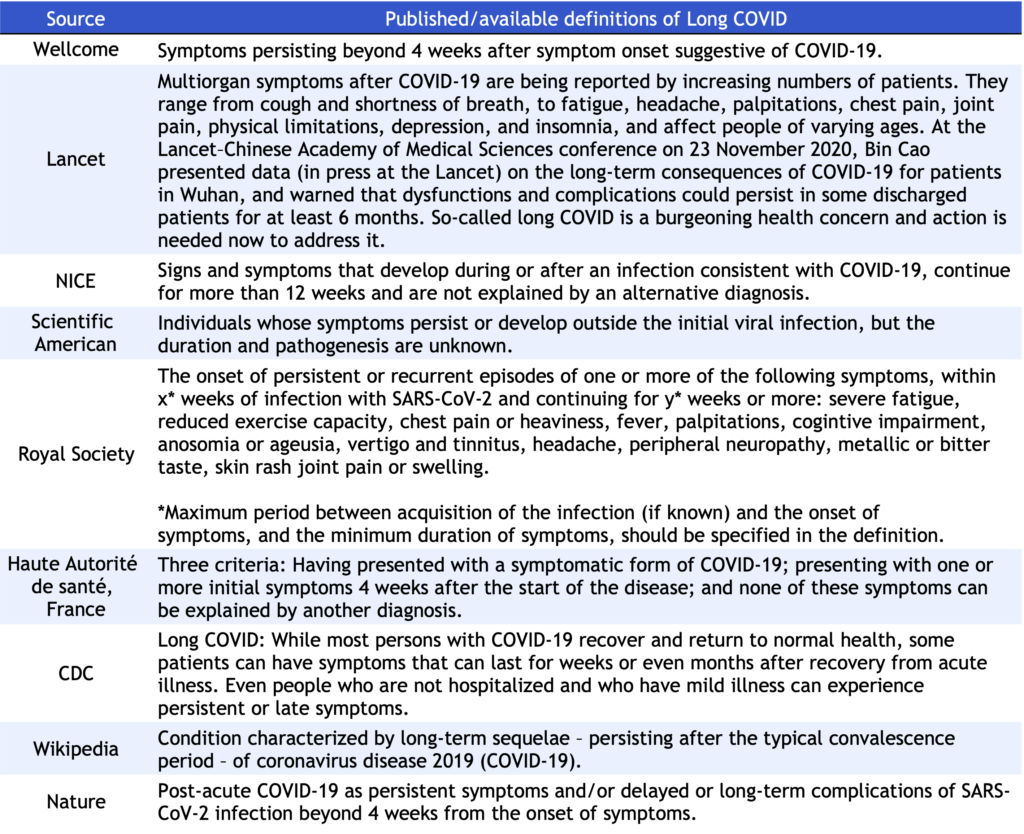
As we surpass the two-year mark of the COVID-19 pandemic, Long COVID has become a challenging global health threat. To meet the needs of a growing population of Long COVID patients, the CDCN is expanding the scope of the CORONA project to develop a Long COVID open-source data repository, similar to STORM for acute COVID. While strategizing to identify and track treatments being used for Long COVID patients, we immediately noticed the glaring paucity of research as compared to acute Covid-19, leaving a wide gap in knowledge of prevalence, incidence, underlying mechanism, clinical manifestations, outcomes, and treatments of the disease. The current plateau in scientific advances in Long COVID treatments may partially be explained by the absence of standardized terminology and definition of the disease. A clear example of heterogeneity in disease terminology and definition is demonstrated below in published definitions of Long COVID, compiled by WHO (https://apps.who.int/iris/bitstream/handle/10665/345824/WHO-2019-nCoV-Post-COVID-19-condition-Clinical-case-definition-2021.1-eng.pdf) (Table 1).
Table 1. Published/available definitions of Long COVID, compiled by WHO.
In alignment with the goals of the CORONA project, this article aims to:
- Provide a brief overview of the heterogeneity in Long COVID terminology and definitions as seen in registered clinical trials,
- Discuss its implications in clinical trials, and
- Urge the establishment of a ubiquitous working definition.
What we found
We searched for clinical trials in all phases using ClinicalTrials.gov and the curated database of all COVID-19 trials maintained by the WHO. The search for trials measuring drug efficacy on Long COVID patients yielded 78 clinical trials.
Different terms for Long COVID
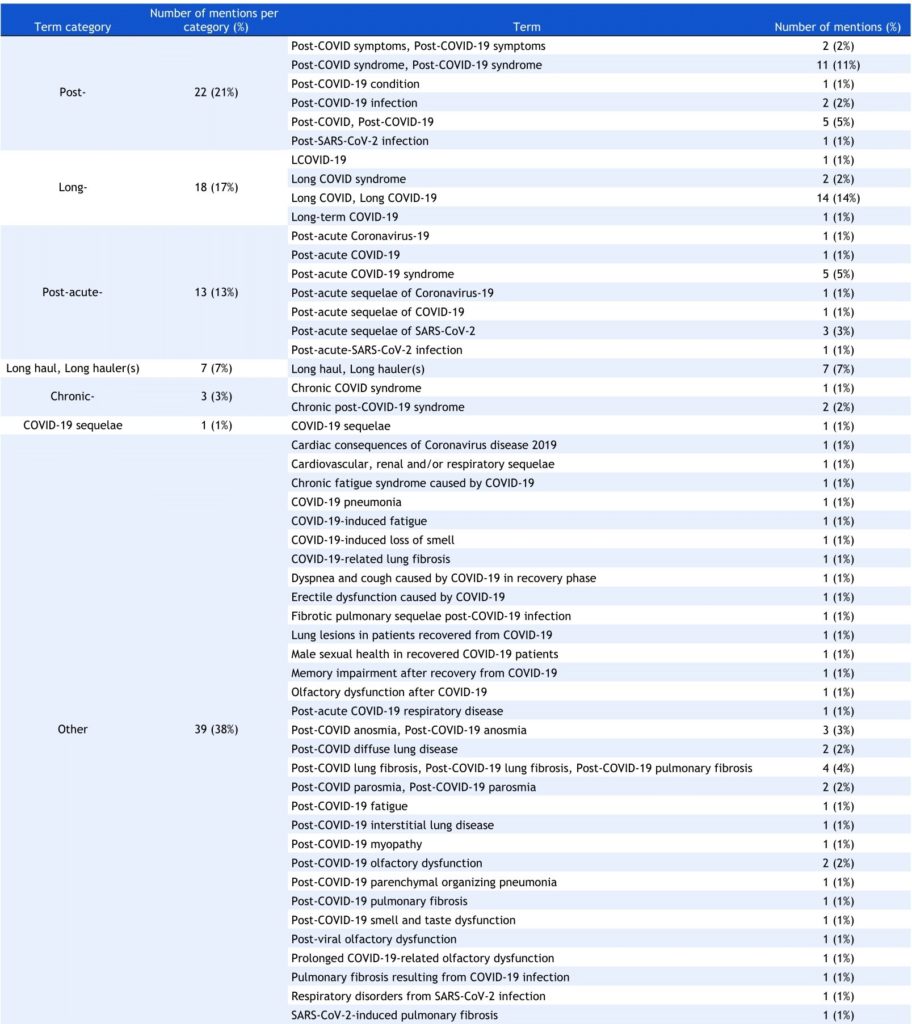
To examine the heterogeneity in terminology, we collected all terms suggestive of Long COVID or various symptoms and conditions associated with the disease across all 78 trial registrations. Illustrated in Table 2, we found 52 unique iterations of the disease name. To better quantify these comparisons, we classified all 52 terms into 7 categories based on common characteristics. Most frequently used terms began with the prefix “Post-,” which constituted 21% of all terms. Next, terms beginning with “Long-” (17%) and “Post-acute-” (13%) were second and third most used, respectively. The individual terms belonging to each category are further displayed in Table 2. Lastly, the “Symptom-specific” category captured all terms referring to one or more Long COVID-associated symptoms and conditions (e.g. “Post-COVID myopathy” and “COVID-19-induced fatigue”) and accounted for 38% of collected terms.
Table 2. Terms used to refer to Long COVID found in trial registrations.
Different definitions and characterizations of Long COVID
No clinical trials provided a comprehensive definition of Long COVID. However, in order to characterize Long COVID, some trials (n=23, 29%) identified the minimum duration of symptoms to which the terms “Long COVID” or “persistent symptoms of COVID” apply in their eligibility criteria (Table 3). In other words, the minimum duration of symptoms refers to the minimum length of time a patient must be experiencing symptoms following an acute infection to be diagnosed with Long COVID. The minimum durations of “persistent symptoms” varied over the range of 4 weeks (n=9) and 12 weeks (n=11). The complete list of trials and their specified minimum duration are shown in Table 3.
Table 3. Minimum durations of persistent/lingering symptoms found in trial eligibility criteria.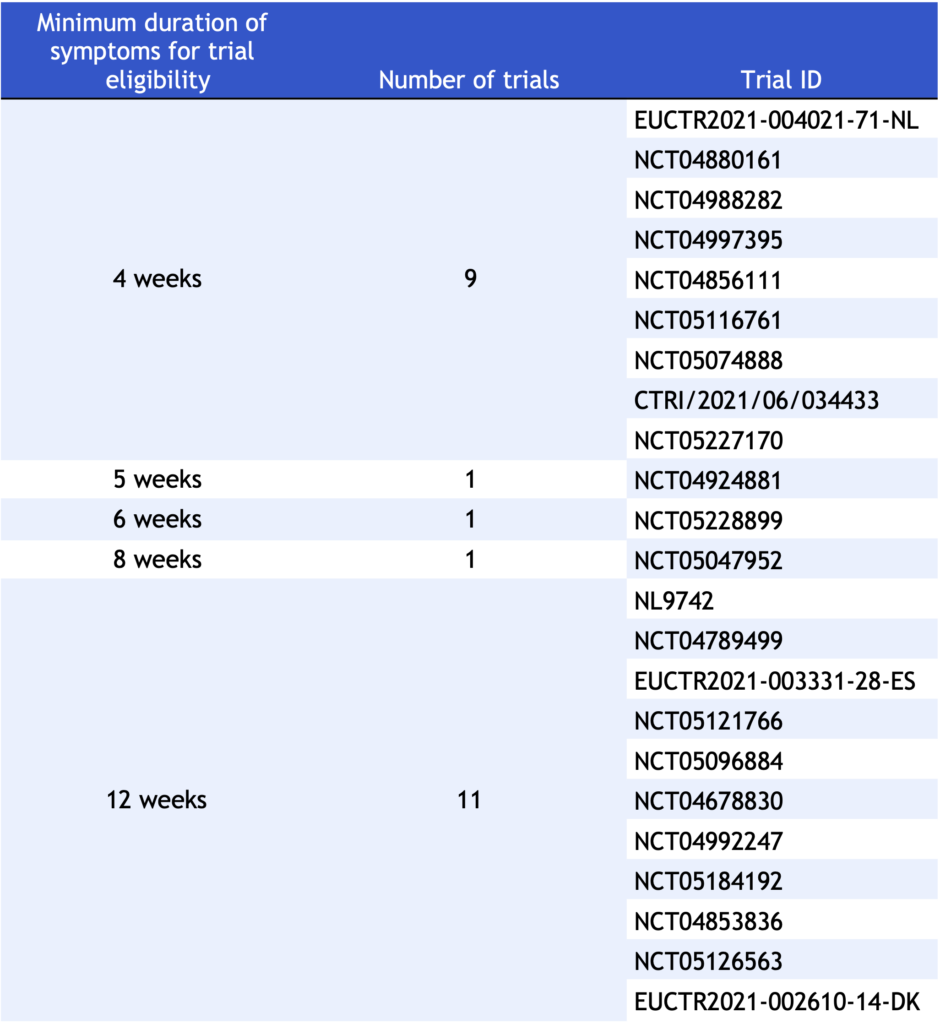
Implications in clinical trials
This review reveals the current utilization of different terminology in 78 registered clinical trials and further emphasizes the need for standardized language to define Long COVID as an entity. Not only are there 52 unique names for the same disease, but the disease definitions also differ greatly, ranging from 4 weeks to 12 weeks. Standardized terminology, characterization, and definition are especially crucial in designing therapeutic clinical trials. It supports clear inclusion and exclusion criteria that ensure reliability and reproducibility of study results. Well-defined patient populations should comprise individuals most likely to benefit from treatment and who best demonstrate its efficacy and safety. Additionally, well-defined exclusion criteria protect vulnerable populations from potential harms and exploitation. A universal definition ultimately allows for appropriate comparisons across multiple trials for the same disease. In sum, the observed heterogeneity in terminology and definition of Long COVID inevitably devitalizes trials and jeopardizes their scientific rigor with unpredictable biases.
As the scientific community continues to make novel discoveries in acute COVID, understanding and defining Long COVID is complicated and difficult. Our upcoming publication will detail relevant challenges and ambiguities further. In the meantime, as the need for internationally agreed-upon terminology is only growing, we hope this review will serve as a catalyst for the adoption of a common language in recognizing Long COVID, particularly in defining the disease. This common language must aim to serve pragmatic purposes dictated by cumulative clinician and patient experiences and be adaptive to the evolving nature of the pandemic. Proper definition is a foundational element in achieving effective therapies through high-quality trials and will contribute significantly to global effort in managing Long COVID.
The next steps for the CORONA project include providing the latest information and resources on Long COVID trials through a public viewer and publishing a detailed review exploring the complexity of defining Long COVID.
Read additional articles about the CORONA Project and its findings in our CORONA INSIGHTS blog.
Disclaimer:
CORONA Insights and the CORONA Treatment Registry are for informational and exploratory purposes only and are not intended to be used as medical advice. This site is intended to facilitate the exploration of therapies mentioned in COVID-19 medical literature. Users assume full responsibility for use of the information on this site and understand and agree that the CDCN and its third party content providers are not responsible or liable for any claim, loss, or damage (including personal injury or wrongful death) resulting from its use. Reliance on any information provided by the CDCN or third party content providers is solely at your own risk. Our extractors do not assess the scientific merits of these results and conclusions. We disclaim any warranty concerning the accuracy, timelessness, and completeness of information on this site. This site also contains links to external sites. The CDCN is not responsible for the content and does not make any representations regarding their content, timeliness, or accuracy.